 Business is brisk in the lab of John Wherry and his team from the Department of Microbiology and the Institute for Immunology for papers on killer and helper T cells. Two studies -- bound by their focus on transcription factors important in the immune response – have come out of the lab in the last few weeks. And, they both identify potential new targets for cancer immunotherapies.
Business is brisk in the lab of John Wherry and his team from the Department of Microbiology and the Institute for Immunology for papers on killer and helper T cells. Two studies -- bound by their focus on transcription factors important in the immune response – have come out of the lab in the last few weeks. And, they both identify potential new targets for cancer immunotherapies.
Transcription factors are proteins that attach to specific DNA sequences in the nucleus to control availability of DNA to be transcribed into messenger RNA. These factors control the differentiation of killer T cells -- whose job is to eliminate infected cells or tumor cells, as well as the support crew helper T cells. This type of T cell coordinates other arms of the immune response including sustaining killer T cells and aiding B cells in making antibodies.
The lab’s paper in Immunity “was a genomic-scale profiling of what prevents T cells from responding to persisting infections or cancers,” says Wherry. The main players in the profile are sets of surface proteins on helper and killer T cells that prevent them from recognizing tumor or infected cells as “other.” Collectively, these proteins are called checkpoints in the immune response, and the team found that certain transcription factors play a larger role than first expected in the intricacies of the immune response.
Molecules that inhibit checkpoints of T-cell activation like the receptor PD-1 are being used to treat melanoma, other cancers, and persisting infections, either as an initial therapy or after relapse. These approaches help restore effective T-cell responses and have been a major breakthrough in cancer immunotherapy with response rates in clinical trials of 30 to 50 percent.
However, a major limitation to this approach is T-cell exhaustion from chronic exposure to tumor antigens, which activates other inhibitory receptors on T-cell surfaces. Blocking more than one of these immune checkpoints can rescue otherwise exhausted antitumor T cells, and this current study identifies a set of new potential targets for cancer immunotherapies.
Overcoming T-cell exhaustion – a major research topic in the Wherry lab -- is also important for combating chronic infections. After an infection, memory T cells persist and can rapidly perform what immunologists call effector functions such as secreting antiviral proteins or directly killing infected cells and expanding upon reinfection. But in persisting infections, these T cells also become exhausted and lose those functions.
Although helper T cells also play a role in chronic infection and cancer, the effect of a persisting infection on helper T-cell function is less well understood. Robust helper T-cell responses are a critical feature of antiviral immunity because these cells can prevent killer T-cell exhaustion during chronic viral infections.
The Penn team defined the molecular profiles of exhausted helper T cells. These cells had a profile distinct from effector and memory helper T cells and also from exhausted killer T cells, although exhausted helper and killer T cells shared some common features, including some of the major checkpoint pathways like the receptor protein PD-1.
“We demonstrated unappreciated roles for certain transcription factors during helper T-cell exhaustion,” says Wherry. “What’s more, the signature of helper T-cell exhaustion was distinct from that of other types of helper T cells, such as those that respond to parasitic infection, or that help make antibodies. This study provides a framework for therapeutic interventions targeting exhausted helper T cells in cancer and chronic infections where improving the function of these cells may have broad impact on the other aspects of immunity they coordinate.”
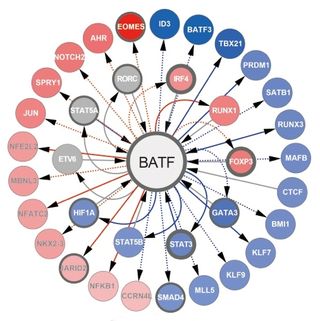
The Wherry lab also published a paper in Nature Immunology this month about another transcription factor called BATF, an essential checkpoint in the development of effector killer T cells.
BATF starts the process of differentiation in “naïve” cells that will eventually become effector killer T cells, which can be activated by an infection or vaccine. “Without BATF, the process of T-cell maturation is a big mess,” say Wherry.
Killer T cells without BATF showed profound defects in expanding after an infection and problems with basic cellular function resulting in a rapid arrest of cell division, failure to use nutrients, and extensive cell death soon after encountering a pathogen.
BATF amplifies T-cell dependent expression of transcription factors and adds to the propagation of inflammatory signals but paradoxically restrains the expression of genes encoding effector molecules. This checkpoint prevents irreversible commitment to an effector fate for cells until a critical threshold of downstream transcriptional activity is met within other cells.
“BATF may be a critical checkpoint to target when optimal T-cell activation is required, that is for vaccines or therapies for cancer, or may be useful to inhibit when a complete shut off of a T-cell response is needed such as in autoimmune scenarios,” notes Wherry.