PHILADELPHIA – A new appreciation for the interplay between two cell nucleus proteins that lead both intertwined and separate lives is helping researchers better understand fatty liver disease, according to a new study by researchers at the Perelman School of Medicine at the University of Pennsylvania. They published their findings this month in Genes & Development.
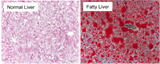
Normal mouse liver and a liver full of fat, stained with a dye called "Oil Red O" where fat shows up as red.
Credit: Courtesy of the lab of Mitch Lazar, Perelman School of Medicine, University of Pennsylvania
The liver normally makes and stores fat, which is required in moderation for normal body function. However, excess fat can cause major liver damage. In fact, fatty liver is a leading cause of liver failure in the United States and is often brought on by obesity and diabetes.
“We showed that simply deleting the protein HNF6 in liver cells of adult transgenic mice fed normal laboratory chow leads to fatty liver syndrome,” said senior author Mitchell Lazar, MD, PhD, director of the Institute Diabetes, Obesity, and Metabolism. “Although HNF6 is known mainly as an activator of protein transcription, loss of it upregulates many lipid-producing genes.”
This loss of HNF6 affects another regulatory protein in the nucleus called Rev-erb-alpha, which also lifts the brakes off the production of fats, thereby also leading to a liver full of fat-filled cells. “When we took HFN6 out the liver, Rev-erb-alpha didn’t know how to get to many of the places that it usually goes to in the liver cell genome,” Lazar said.
Many of these lipid-producing genes are targets of Rev-erb-alpha, which plays a central role in coupling metabolism to the circadian clock, and binding of Rev-erb-alpha at these sites was lost when HNF6 was made inactive in liver cells. Normally, HNF6 delivers Rev-erb-alpha to parts of the genome it doesn’t find on its own. If HNF6 is gone, Rev-erb-alpha’s gene inactivation function is turned off – essentially suppressing a repressor. In this way HNF6 and Rev-erb together regulate aspects of liver lipid metabolism, but they also each independently affect other sets of genes in the liver and other tissues.
Lazar likens this control of normal liver physiology over a 24-hour cycle to the building game Jenga. “We are still trying to figure out which blocks are needed and which ones are not needed to keep the tower, or healthy liver, standing, as it were,” he said. “If we take out the ‘blocks’ Rev-erb-alpha or HNF6 from the liver ‘tower,’ what happens? What this study tells us is that these two blocks are co-dependent in some situations.”
Rev-erb-alpha governs the daily cycle of many genes that play a role in maintaining normal metabolic cycles in many tissues. An integrated view of normal metabolism is important to be able to see what happens during abnormal metabolism in order to know how to maintain healthy pathways and at the same time how to fix the ones that have gone awry.
Co-authors are Yuxiang Zhang, Bin Fang, Manashree Damle, Dongyin Guan, Zhenghui Li, and Yong Hoon Kim, all from Penn, and Maureen Gannon, from Vanderbilt University.
This work was supported by the National Institutes of Health (R01 DK45586, U01 DK089540, R01DK105689) and the Cox Medical Research Institute.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.