PHILADELPHIA –Studies at the University of Pennsylvania School of Medicine on brain electrical signaling offer a fresh perspective on vertebrate evolution, provide additional evidence supporting Darwinian views of evolution, and may also lead to more effective treatment of epileptic seizures in infants. Researchers discovered how evolutionary changes produced a series of improvements in molecules generating electrical signals in nerves between 550 and 400 million years ago. By making nervous systems faster and smarter, these innovations appear to have contributed to the evolutionary success and diversity of vertebrate animals.
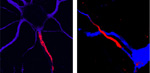 |
Neurons from the sea lamprey (right) and mammalian brain (left), shown in blue. Strong red labeling shows the location of clustered sodium ion channels that initiate electrical impulses at the beginning point of nerves in both lampreys and mammals.
Click on thumbnail
to view full-size image |
In an evolutionary comparison of nerve cell genes appearing in PLoS Genetics last month, Penn scientists show that improvements in the molecules that govern rapid nerve impulses occurred at major turning points in evolutionary history. By making nerve signals faster and more controllable, these innovations appear to have contributed to the building of smarter brains, and perhaps even to the success and diversity of vertebrates. In other experiments presented at the Society for Neuroscience meeting in November and soon to appear in the Annals of Neurology, the scientists found that the same electrical signaling molecules appear to be an effective target for anti-seizure drugs for human newborns.
The electrical signaling molecules at the center of both studies are two related types of nerve cell proteins called sodium and potassium channels. A decade ago, researchers found that mutations in genes for these molecules were a cause of some forms of epilepsy in newborn babies and infants. Sodium channels were already targets of anti-epileptic drugs. The team led by Assistant Professor of Neurology Edward C. Cooper, MD, PhD, focused on the potassium channels for therapeutic development.
Epilepsy is a common condition in which seizures, involuntary attacks of loss of awareness and bodily control, are experienced recurrently. Epilepsy can begin at any age, but incidence is highest in the vulnerable first few weeks of life and remains elevated in later infancy and early childhood.
Initial work by the Penn team showed that the potassium and sodium channels were clustered together in small patches on the long fibers, called axons, which transmit electrical impulses between nerve cells. This raised several questions from the both the evolutionary and clinical underpinnings of this line of research: First, how did these two types of channels evolve to become so tightly paired at these patches on nerves? Second, is the development of these clusters over time important for understanding how channel mutations cause epilepsy? Third, clinically, since the potassium channel mutations linked to newborn epilepsy decreased channel activity, could drugs that increased the potassium channel activity be effective for seizure prevention?
Anchoring Sequences
Sodium and potassium channels are proteins embedded in the nerve membrane, with a part of each channel exposed to the cell’s interior. In 2006, Cooper’s team showed that the intracellular parts of the potassium and sodium channels possessed similar amino acid sequences. The shared sequences contained instructions specifying that the channels should be anchored together at spots along the axon.
They also found that these anchoring sequences were conserved in the potassium and sodium channels of vertebrates over 350 million years of evolution, from fish to humans. However, the channels of invertebrates, including fruit flies, worms, and squids, lacked the clustering sequences. In addition, some mutations causing epilepsy in infants prevented the channels from assuming their clustered positions within the patches.
“Finding that the sodium and potassium channel clustering required nearly identical sequences that seemed to have evolved at the same time was very surprising” said Cooper. “The similarity in anchor sequences could not arise by chance. Seeing them in both channel types was comparable to a person arriving at a new job, and seeing that their new coworker wore exactly the same clothes, drove the same model and color of car, and had a spouse and children with the same names. It seemed inexplicable.”
To solve the mystery, the researchers studied the channels of classes of animals that evolved earlier than vertebrates, including sea urchins and primitive organisms related to vertebrates called lancelets, sea squirts, and lampreys. The project received a boost, because the genomes of each of these organisms are currently being sequenced, supported by the National Human Genome Research Institute. Analysis of the channels from these primitive organisms solved the mystery.
The Rise of the Sodium Channel
The new findings, reported in the December 26th issue of PLoS Genetics, show that the sodium channel anchor sequence arose much earlier than previously known, at perhaps the most important turning point in biological history, the beginning of the so-called Cambrian Explosion (550-530 million years ago). During the Cambrian Explosion, all the major groups (or phyla) of animals alive today suddenly – in an evolutionary time scale – appeared. The new analysis showed that all members of the phylum Chordata, which includes lancelets, sea squirts, and lampreys, as well as vertebrates, have sodium channels with the anchor sequence.
The sodium channels of nonchordates (including invertebrates such as insects and mollusks) lacked this clustering sequence. This is important because sodium channel clustering makes nerve signaling much more rapid, reliable, and energy-efficient. As chordates evolved into vertebrates, they relied on the sodium channel clusters as components of increasingly complex systems for sensation, brain computation, and control of body movement. All other animals, lacking the ability to generate such rapid signals, generally relied on smaller body size and simpler nervous systems.
Potassium Channels as Shock Absorbers
Potassium channel clustering arose later and complements sodium channel signaling. Unlike sodium channels, none of the potassium channels of very early chordates had the clustering sequence. Potassium channel clustering sequences first appeared in a vertebrate ancestral to sharks, “only” around 400 to 450 million years ago.
The clustered potassium channels serve an important supporting function, making nerve signals that are started by sodium channels more controllable. “Sudden openings by a lot of sodium channels clustered together can be jarring and unpredictable for the nerve cell, like a car hitting a bump in the road.” says Cooper. “The potassium channels are like the shock absorbers on the car’s suspension—they dampen some of the oscillations caused when the sodium channels open and calm the nerve, just as shock absorbers reduce bouncing and help maintain control on a bumpy road.” The fact that this combination of channels clustered together on nerves has been completely conserved for about 400 million years in all types of vertebrates underlines its importance, as does the fact that epilepsy results when the clusters are disrupted.
In related studies, the researchers tested whether a drug that causes increased openings by the axons’ clustered potassium channels might be effective in preventing seizures in newborns. They found that an opener, called flupirtine, was more powerful in preventing seizures than either of two drugs now in frequent clinical use, phenobarbital and diazepam. These experiments used established models of neonatal epilepsy, performed on immature rats. Next steps include additional tests of the safety of flupirtine in immature animals, and then, consideration of possible trials in human infants.
The Need for Speed
In an often-quoted section of the Origin of Species, Charles Darwin expressed his complete inability to explain why such a diversity of animals appeared so rapidly in the Cambrian period, the so-called Cambrian Explosion. Scientists have more recently advanced theories for this based on changes in the Earth’s environment, or evolution in the genes controlling animal body form. The new PLoS Genetics study, suggests Cooper, points to an additional factor that might come into play: evolutionary changes to the nervous systems of early animals, such as sodium channel clustering, which led to new behaviors such as improved swimming and predation.
This research provides a vivid example of the ability of evolution to build very complex structures through a series of incremental steps. “The vertebrate axon is elegant and extremely intricate, with a central fiber contributed by a neuron and a jelly-roll like myelin wrapping made by other cells called glia,” says Cooper. It was previously unknown which came first, the channel clustering on the neuron, or the myelin wrapping.
“Our studies show that the sodium channel clustering evolved first, perhaps 100 million years before myelin,” says Cooper. “Myelin appeared, like potassium channel clustering, in an ancestor of the shark. This interval provides plenty of time for the molecules necessary for myelination to evolve. Each step made the nerve faster, and more reliable and efficient, because the sodium channel clusters were already present on the axon.”
This work was funded by the National Institute of Neurological Diseases and Stroke, The Roy and Diana Vagelos Scholars Program, The Human Frontiers Science Program, and the Miles Family Fund.
Penn co-authors are Alexis Hill, Guixin Zhang, Michael Selzer and David Lapides. Yogendra Raol and Amy Brooks-Kayal of Children’s Hospital of Philadelphia (now at the University of Colorado) coauthored the flupirtine therapy study. Koichi Nakajo of the Japanese National Institute for Physiological Sciences, and Atsuo Nishino and Yasushi Okamura of Osaka University co-authored the channel evolution study.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to most recent data from the National Institutes of Health, received over $379 million in NIH research funds in the 2006 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s top ten “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.