Closing In on the Cellular Culprits of Schizophrenia
(Philadelphia, PA) – The cause of schizophrenia
remains a mystery, despite the millions of dollars spent
trying to discover which genes play a role in its etiology.
In at least 10 populations around the world, a significant
association between schizophrenia and the gene for dysbindin
has been noted – making dysbindin the most highly
replicated schizophrenia-associated gene described to
date. Now, researchers at the University of
Pennsylvania School of Medicine are starting
to place where dysbindin fits in the pathway that leads
from a gene to a psychiatric disorder. Schizophrenia
affects between 1 to 2 percent of people worldwide during
their lifetime and about 2.2 million American adults
have schizophrenia in a given year.
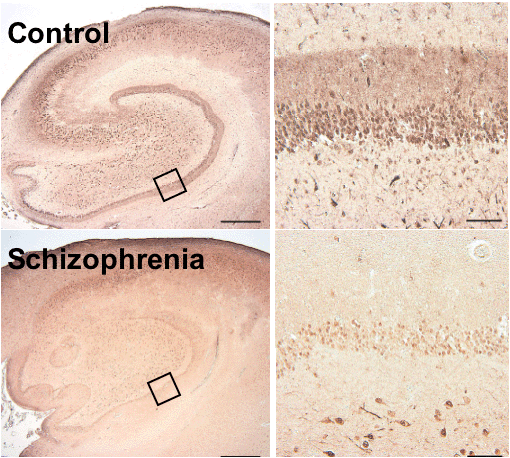 Using quantitative immunohistochemistry in postmortem
brain tissue, the Penn investigators found that the
expression of dysbindin protein was reduced in more
than 80 percent of the patients with schizophrenia by
an average of 40 percent relative to matched healthy
controls.(Click on the thumbnail image above to view
the full-size photo). “This is among the most
significant findings I’ve seen yet in schizophrenia
postmortem research, and it represents a critical lead
for understanding schizophrenia,” says senior
author Steven Arnold, MD, Associate
Professor of Psychiatry and Neurology. The research
appears in the May issue of the Journal of Clinical
Investigation.
Using quantitative immunohistochemistry in postmortem
brain tissue, the Penn investigators found that the
expression of dysbindin protein was reduced in more
than 80 percent of the patients with schizophrenia by
an average of 40 percent relative to matched healthy
controls.(Click on the thumbnail image above to view
the full-size photo). “This is among the most
significant findings I’ve seen yet in schizophrenia
postmortem research, and it represents a critical lead
for understanding schizophrenia,” says senior
author Steven Arnold, MD, Associate
Professor of Psychiatry and Neurology. The research
appears in the May issue of the Journal of Clinical
Investigation.
The scientists also found that, in the same brain regions
in which there was a decrease in dysbindin, there was
also an increase in the amounts of presynaptic glutamate
packets, or vesicles, and that these findings were highly
correlated. Synaptic vesicles form at the ends of nerve
cells and contain chemical neurotransmitters such as
glutamate. Neurons communicate with each other by releasing
neurotransmitters from these vesicles. The researchers
surmise that dysbindin affects the manufacture or breakdown
of these vesicles and, consequently, glutamate may not
be released properly – thus impairing communication
between neurons.
The abnormality was most prominent in the dentate gyrus
portion of the hippocampus. This area of the brain is
especially important for memory, which is known to be
impaired in schizophrenia. The study’s findings
were independently replicated, using two collections
of postmortem brain tissue, one maintained by Penn and
another by the Stanley Medical Research Institute.
“The next step is to understand what dysbindin
does in the brain,” explains Arnold. “We’ve
found that dysbindin abnormalities are part of schizophrenia,
but we need to know much more to translate this information
into practical knowledge to help patients. In other
words, we need to know what other proteins dysbindin
interacts with and whether it involves just glutamate
or other neurotransmitters like serotonin, dopamine
or GABA and how dysbindin affects the electrical activity
of the brain. And, are there medicines that alter dysbindin
expression in the brain?” To answer these and
other important questions, Arnold and colleagues are
currently collaborating with other researchers at the
University of Oxford in the United Kingdom.
“One of the most exciting parts of this story
is that the extensive work that has gone on in the genetics
of schizophrenia is finally starting to bear fruit in
terms of identifying specific genes that we can then
follow-up in the brain,” says Arnold. “Who
would have predicted that a protein that was first discovered
a few years ago by muscular dystrophy researchers could
have anything to do with schizophrenia? The genetics
studies pinpointed a link between dysbindin and schizophrenia.
This clue prompted us to investigate dysbindin in the
brain where we found that it is highly expressed and
highly abnormal in schizophrenia.”
Other Penn researchers collaborating on this work are
Konrad Talbot, Wess L. Eidem, Edward W. Thompson, Rachel
J. Smith, Chang-Gyu Hahn, John Q. Trojanowski, and Raquel
E. Gur, as well as Caroline L. Tinsley and Matthew A.
Benson from Oxford University. The research was supported
in part by the National Institute of Mental Health.
For
a printer friendly version of this release, click
here.
###
PENN Medicine is a $2.5 billion enterprise dedicated
to the related missions of medical education, biomedical
research, and high-quality patient care. PENN Medicine
consists of the University of Pennsylvania School of
Medicine (founded in 1765 as the nation’s first
medical school) and the University of Pennsylvania Health
System (created in 1993 as the nation’s first
integrated academic health system).
Penn’s School of Medicine is ranked #2 in the
nation for receipt of NIH research funds; and ranked
#4 in the nation in U.S. News & World Report’s
most recent ranking of top research-oriented medical
schools. Supporting 1,400 fulltime faculty and 700 students,
the School of Medicine is recognized worldwide for its
superior education and training of the next generation
of physician-scientists and leaders of academic medicine.
Penn Health System consists of four hospitals (including
its flagship Hospital of the University of Pennsylvania,
consistently rated one of the nation’s “Honor
Roll” hospitals by U.S. News & World Report),
a faculty practice plan, a primary-care provider network,
three multispecialty satellite facilities, and home
health care and hospice.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.