 A new study is shedding light on how cells respond to surrounding “stiff” tissue that can influence cells to go rogue—and ultimately cause disease. A better understanding of that process could help advance the development of treatments for cancers and cardiovascular disease resistant to therapy.
A new study is shedding light on how cells respond to surrounding “stiff” tissue that can influence cells to go rogue—and ultimately cause disease. A better understanding of that process could help advance the development of treatments for cancers and cardiovascular disease resistant to therapy.
An article in Science Signaling describes a pathway that cells use to convert information about the stiffness of proteins that surround them to the inner depths of the cell, a process that can influence cell replication and movement.
Cells not only sense and respond to chemical cues, they also respond to the stiffness or flexibility of the tissue around them, collectively called the extracellular matrix (ECM). ECM stiffness can determine whether a cell proliferates or stays quiescent. The exact response depends on the type of tissue the ECM touches and also if the organ or tissue is diseased, injured, or healthy. The ECM is stiff in unhealthy blood vessels, for example. Arterial stiffening has long been considered a major risk factor for cardiovascular disease. Keeping arteries soft and supple might reduce disease risk, researchers surmise.
Proteins called integrins and several of their associated adhesion proteins are associated with sensing ECM stiffness. In this new study,
Richard K. Assoian, PhD, professor of Pharmacology and director of the Program in Translational Biomechanics for the Institute of Translational Medicine and Therapeutics at Penn, and
Yong Ho Bae, PhD, an American Heart Association post-doctoral fellow in the Assoian lab, investigated how cells interpreted ECM stiffness into physiological changes within the cell.
The findings of this study might be applied to the development of therapeutics that could perhaps limit the unwanted proliferation that can occur in disease when the tissues surrounding cells stiffen. “In cancer and cardiovascular disease, the current thinking is that flexible cells are normal and stiff cells are pathological. In normal wound healing, a transient stiffening may be needed to let cells proliferative and move in order to heal the wound. But then the tissue stiffening should reverse. In disease, the stiffening may be irreversible, leading to pathology,” explains Assoian.
The team found that a pathway involving the enzyme guanosine triphosphatase Rac triggered proliferation of cells growing on a custom-designed ECM-coated surface, but only when the cells were exposed to a stiff ECM.
When they deleted Rac in smooth muscle cells in mice, these cells did not proliferate, and the mice did not efficiently repair damage to injured blood vessels. Since smooth muscle cells in these injured blood vessels "see" the same stiffness as the isolated cells, these results show that Rac is essential to sense ECM stiffness and allow for smooth muscle proliferation during the repair response to injury.
More specifically, they found that the pathway involved a focal adhesion kinase (FAK), the adaptor protein p130Cas (Cas), and Rac, which collectively transduced ECM stiffness into intracellular stiffness, increased the abundance of the cell cycle protein cyclin D1, and promoted S-phase entry. These three changes are likely to be pro cell proliferation in cancer as well as cardiovascular disease.
The Rac-dependent stiffening also involved lamellipodin, a protein that transmits Rac signals to the cytoskeleton during cell migration, a critical process in metastasis as well as cardiovascular disease.
“Our findings establish the FAK-Cas-Rac-lamellipodin signaling path converts external information about ECM stiffness into stable intracellular stiffness and mechanosensitive cell replication,” says Assoian. In this way, lamellipodin is important not only in controlling cellular migration but also for regulating the cell cycle in response to mechanical signals.
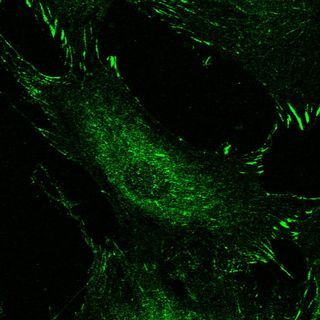
Photo: Signaling complexes (green) concentrated at the edges of replicating cells on stiff surfaces.