It turns out that bitter taste in your mouth isn’t such a bad thing after all.
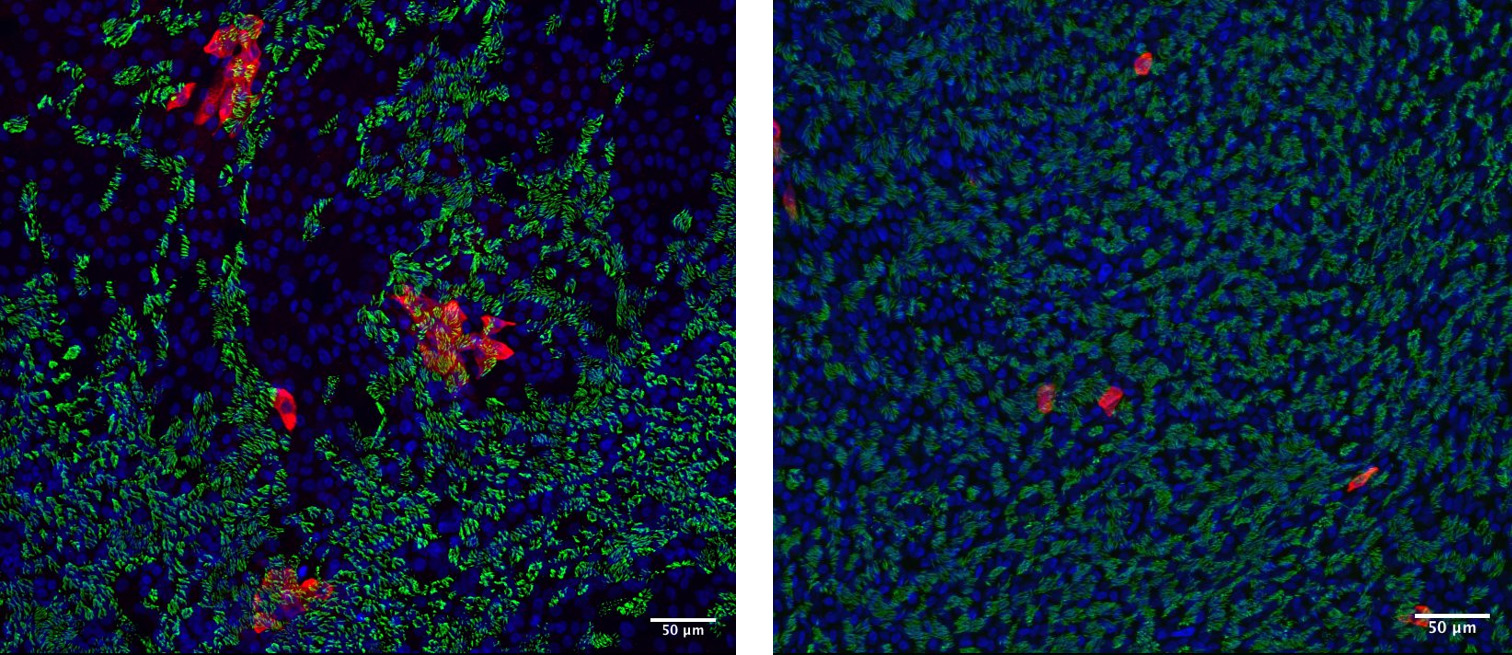 Primary nasal cultures derived from two different individuals infected with SARS-CoV-2. Twenty-four hours after infection, the cultures were stained for virus (nucleocapsid protein – red), motile cilia (green), and nuclei (blue). Cultures from one donor (left) demonstrate clusters of infected cells while cultures from another donor (right) demonstrate single discrete infected cells.
Primary nasal cultures derived from two different individuals infected with SARS-CoV-2. Twenty-four hours after infection, the cultures were stained for virus (nucleocapsid protein – red), motile cilia (green), and nuclei (blue). Cultures from one donor (left) demonstrate clusters of infected cells while cultures from another donor (right) demonstrate single discrete infected cells.
Known as taste family 2 receptors, or T2Rs, bitter taste receptors have become a source of continuing revelation to researchers at Penn Medicine and elsewhere. Using a biobank of cryopreserved human sinonasal cells established by the division of Rhinology (Drs.
Cohen,
Palmer,
Adappa,
Kennedy, and
Thaler), in 2012, the Penn Rhinology research team made the surprising discovery that bitter taste receptors could be targeted for therapeutic intervention. Exploring the capacities of a T2R in the upper respiratory epithelium known as T2R38, the team found it could be activated by gram-negative bacteria to provoke the local production of calcium-dependent nitric oxide (NO).
A highly reactive radical, NO is known to stimulate direct antibacterial effects, as well as increasing mucociliary clearance. In addition to the implications for treatment, these findings established T2R38 as a part of the innate defense mechanism of the respiratory system and found that other T2Rs expressed in the upper airway epithelium regulate NO production.
This diverse biobank of cryopreserved respiratory epithelial cells collected from more than 1,000 patients undergoing rhinology surgery has continued to be used to study the role of T2Rs in the immunology of chronic rhinosinusitis.
 Capable of recapitulating the respiratory epithelium, with its cilia and mucosa, the biobank tissue samples were gathered with data on demographics, comorbidities and taste receptor genotype. Drs. Cohen and Robert Lee, PhD were then able to compare cells from different donors in the context of specific T2R Genotypes. The result? A better understanding of individual infection variability and other factors relevant to respiratory infection.
Capable of recapitulating the respiratory epithelium, with its cilia and mucosa, the biobank tissue samples were gathered with data on demographics, comorbidities and taste receptor genotype. Drs. Cohen and Robert Lee, PhD were then able to compare cells from different donors in the context of specific T2R Genotypes. The result? A better understanding of individual infection variability and other factors relevant to respiratory infection.
With the arrival of COVID-19 in March 2020, however, Dr Cohen and Michael Kohanski, MD, PhD shifted their focus to SARS-CoV-2 and its effects in the sinonasal cavity. In partnership with GeneOne Life Sciences, a clinical trial utilizing topical Quinine to stimulate sinonasal epithelial NO as an anti-microbial therapy for chronic rhinosinusitis (NCT04060316) was about to start, however the trial was placed on hold due to the pandemic. Based on prior work on SARS and NPO the investigators felt the trial could be repurposed to focus on COVID-19 prophylaxis and received approval from the FDA for this new indication (NCT04408183). The study’s supplementary objectives explore the sinonasal environment in the context of SARS-CoV-2 prior to the manifestation of symptoms, and other as-yet-unknown or ill-defined facets of the disease and its effects.
Nitric Oxide and Quinine Bitters
In the wake of the SARS epidemic, an eloquent series of investigations appeared from the Karolinska Institute in Stockholm to demonstrate that nitric oxide has antiviral effects against envelope viruses, a group that includes both SARS-CoV and SARS-CoV-2. The researchers then showed that they could neutralize the first generation of SARS with high levels of NO.
The molecule Drs. Cohen and Lee are using in their investigational spray is quinine, in a concentrate that mimics that of the quinine in tonic bitters.
“Quinine interested us for several reasons,” Dr. Cohen says. “First, it has the advantage of being an FDA-approved agent. Second, we know from a study we completed several years ago that when quinine is sprayed into the nose, it activates ciliary beat frequency and NO production in a way that suggests that for T2R activation.”
COVID-19 and the Known Unknown
Rather than recapitulate what’s known about SARS-CoV-2 from the thousands of clinical reports and observational reports that have appeared in recent months, the Penn Rhinology lab is applying their prior experience to COVID-19 as a point of reference to explore what remains undefined.
 “We know that respiratory viruses gain entry to the body through the nose,” Dr. Cohen said recently. “And we know that SARS-CoV2 is predominantly a ciliated cell infection, followed by viral replication and shedding into the mucus and neighboring cells.”
“We know that respiratory viruses gain entry to the body through the nose,” Dr. Cohen said recently. “And we know that SARS-CoV2 is predominantly a ciliated cell infection, followed by viral replication and shedding into the mucus and neighboring cells.”
But what happens next is in the realm of the known unknown—the mysteries that researchers are aware of, but have yet to illuminate. For some infected individuals, Dr. Cohen explains, SARS-CoV-2 progresses to lower airway disease. For others, it remains in a sort of limbo, present, but never evoking the signs or symptoms of evident disease.
These asymptomatic carriers are among the greatest mysteries of COVID-19, though the phenomenon has been well described in other viral diseases, including hepatitis, Epstein-Barre and poliomyelitis. Given the uncertainties of transmission in persons who display none of the symptoms of SARS-CoV-2, the various pandemic planning scenarios from the Centers for Disease Control place the percentage of asymptomatic carriers at ~40% of COVID-19 cases.
Elucidating the heterogeneity of individual responses to SARS-CoV-2 is a study objective of a recently funded VA grant in collaboration with Susan Weiss Co-director of the University of Pennsylvania Center for Research on Coronaviruses and Other Emerging Pathogens. In preliminary data, a striking variability appeared in viral infection pattern and viral load infectivity among primary nasal cultures derived from different individuals, suggesting that a negligible infection could coexist with a thriving potential for viral spread. In addition, the investigators were surprised to find that the epithelial barrier remained intact for the first 72 hours of infection, and that cells remained alive up to eight days thereafter—a possible explanation for the lack of rhinological symptoms in COVID-19.
Among the study’s additional objectives:
- To clarify the increasing age and racial disparities evident in COVID-19 severity and mortality by ascertaining how demographic and/or genetic variability influences respond to SARS-CoV-2 infection;
- To ascertain viral co-localization with goblet cells, ciliated cells and basal cells; and
- To indicate whether a specific epithelial cell lineage is more vulnerable to SARS-CoV-2 entry, and whether this is associated with expression levels of ACE2.
The viability of prophylactic bitter therapy for SARS-CoV-2 is suggested in studies in which NO inhibited viral replication and impaired viral fusion and entry into target cells when pre-treated with nitric oxide synthase, an enzyme that produces NO.
The investigators believe that the application of the bitter spray will reduce the number of cells infected with SARS-CoV-2 and reduce the amount of shed virus in apical secretions in a NO dependent manner, compared to saline-treated controls. The study is currently enrolling at Penn Medicine.