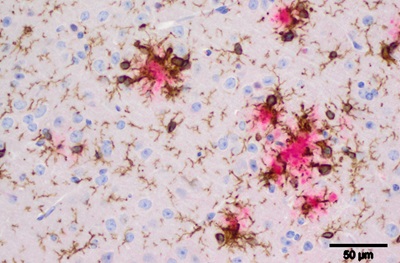
Beta-amyloid plaques from an AD mouse model. Pink represents beta-amyloid deposits (plaques), brown represents microglia cells and blue/purple is nuclei of neurons and glial cells.
Credit: Stefan Prokop, MD, Perelman School of Medicine, University of Pennsylvania
PHILADELPHIA – Three new gene variants, found in a genome wide association study of Alzheimer’s disease (AD), point to the brain’s immune cells in the onset of the disorder. These genes encode three proteins that are found in microglia, cells that are part of the brain’s injury response system. The study is an international collaboration of four AD research consortia that analyzed DNA from 85,000 subjects. The results are reported online this week in
Nature Genetics.
Studies of this type focus on identifying new therapeutic targets for treatment or prevention of AD, a goal of researchers world-wide. Genetic variation of the type described in this paper are “experiments of nature,” of a sort, that reveal when a specific gene is altered, disease risk can be affected.
“This is direct evidence that if drugs can be designed to target these proteins, we have a chance to alter disease risk in people,” said senior author Gerard Schellenberg, PhD, a professor of Pathology and Laboratory Medicine, and director of the Alzheimer Disease Genetics Consortium (ADGC) at the Perelman School of Medicine at the University of Pennsylvania. “It’s been known for decades that microglia — a first-line-of-defense cell we are born with — surround amyloid plaque deposits associated with Alzheimer’s. These multiple gene ‘hits’ all originating from microglia are the clearest demonstration that these cells are part of Alzheimer’s pathology and, more importantly, provide clear protein targets where we can start to intervene with drugs.”
The ADGC, supported by the National Institute on Aging (NIA) at the National Institutes of Health, is one of the four consortia of the International Genomics of Alzheimer's Project on this study. The others are Cohorts for Heart and Aging in Genomic Epidemiology (CHARGE), European Alzheimer’s Disease Initiative (EADI), and Genetic and Environmental Risk in Alzheimer’s Disease (GERAD).
The variants the team found — PLCG2, ABI3, and TREM2 — are all protein-coding mutations in genes that are highly expressed in microglia and are part of an immune cell protein network where multiple components contribute to AD risk. One of the genes, PLCG2, is an enzyme that is a potential drug target.
Key questions remain in how microglia should be targeted and whether the injury response should be inhibited or activated and at what stage of disease. “Since prevention is a key goal of therapy, influencing microglial cells before onset of cognitive changes needs to be explored,” Schellenberg said.
The three variants they identified are fairly rare and he accounts for their success in finding them to their three-stage study. In the first stage, the entire protein coding regions of 34,290 samples were sequenced. In the second and third stages, the team further refined the sequences of variants and verified the significant hits against untested samples from AD patients.
“Our findings show that microglia and the innate immune system -- via microglia -- directly contribute to susceptibility of late-onset Alzheimer’s disease, and are not just a down-stream ‘after-the-fact’ consequence of damage to the brain,” Schellenberg said.
Work at Penn is funded through the NIA (UO1AG032984). Penn coauthors are Amanda Kuzma, Otto Valladares, Liming Qu, Yi Zhao, John Malamon, Beth Dombroski, Laura B. Cantwell, Adam C. Naj, Steven D. Arnold, John Q. Trojanowski, and Vivianna M. Van Deerlin. The ADGC members are Schellenberg and coauthor Li-San Wang at Penn, Richard Mayeux at Columbia, Margaret Pericak-Vance at the University of Miami, , Lindsay Farrer at Boston University, and Jonathan Haines at Case Western Reserve University.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.