PHILADELPHIA – For years, researchers have known that under normal conditions, the breast cancer protein BRCA1 orchestrates the repair of damaged DNA, but the details of just how BRCA1 moves to the damaged site and recruits the right nuclear repairmen for DNA restoration remains a mystery.
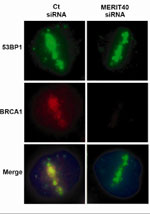 |
BRCA1 (red) readily localizes to laser-induced DNA damage sites demarcated by the DNA repair protein (green) in control cells (left column). BRCA1 recruitment to DNA damage sites is strongly reduced in MERIT40-deficient cells (right column)
Click on thumbnail
to view full-size image |
Now, a new study from the University of Pennsylvania School of Medicine has identified genes associated with the BRCA1 protein and their involvement in the DNA repair pathway, helping to clear the way for researchers to better understand what goes wrong when the BRCA1 gene is mutated and the repair pathway goes haywire. Identifying patients with mutations in these BRCA1-associated genes may help better fight breast cancer.
The new study appears in the most recent issue of Genes & Development.
“A mutated BRCA1 gene increases vulnerability to breast and ovarian cancers by increasing the rate at which genes are altered,” says Roger Greenberg, MD, PhD, Assistant Professor of Cancer Biology at the Abramson Family Cancer Research Institute at the University of Pennsylvania. Previous studies have shown that mutated BRCA1-associated proteins also increase the risk of breast cancer, implying that a BRCA1-centered DNA repair pathway is necessary to suppress cancer.
About two years ago, Greenberg helped to lead a study that identified the role of a BRCA1-associated protein called RAP80. The BRCA1 protein works in partnership with RAP80 to locate damaged DNA sites. Cancer-causing mutant BRCA1 proteins fail to pair up with RAP80, which, in turn, blinds BRCA1 to DNA damage. The results of the study led Greenberg’s group to look closer at the interaction between BRCA1 and RAP80 to learn the molecular details of how this complex functioned in DNA repair and to explore RAP80 as a possible breast-cancer-susceptibility gene candidate.
In the latest study, Greenberg’s team returned to the BRCA1-RAP80 complex for additional analysis, and identified another protein called MERIT40 that works with BRCA1-RAP80 to achieve DNA repair. MERIT40, a previously unknown protein, was revealed by purifying BRCA1-RAP80 complexes from human cells. MERIT40 not only recruits BRCA1 and RAP80 to the sites of DNA damage and signals for DNA repairman to the site, but also acts as the molecular scaffold for the BRCA1-RAP80 complex.
“The BRCA1-RAP80 protein complex works as machine. If a piece of the machine is missing, the machine will begin to break down and eventually stop working,” says Greenberg. Without MERIT40, Greenberg’s group discovered that the BRCA1-RAP80 complex degrades, denying BRCA1 access to the DNA damage sites within the genome.
The identification of MERIT40 as a BRCA1-associated gene may also lead researchers to other genes that increase vulnerability to breast cancer. Greenberg said the identification of the fundamental role of RAP80 several years ago has led researchers to explore RAP80 as a cancer-causing gene. Greenberg said ongoing studies have confirmed that mutations to the RAP80 gene occur in human populations and may cause cancer. These reports will be published later this year, he said.
“There are many genes associated with BRCA1 that turn out to be mutated in breast cancer,” Greenberg said. “MERIT40 now becomes another good gene to study.”
Understanding how BRCA responds to damaged DNA can also help scientists to better fight cancer with chemotherapy, notes Greenberg. By shutting MERIT40 genes off, researchers may be able to weaken cancer cells, leaving them more susceptible to attack by chemotherapy.
In the future, Greenberg says he hopes to gain a better understanding of how MERIT40 works by looking for MERIT40 mutations in breast cancer patients.
The National Cancer Institute, the Sidney Kimmel Foundation Scholar Award, The Mary Kay Ash Foundation Translational Innovation Award and the Abramson Family Cancer Research Institute funded this research.
Study co-authors include: Genze Shao, Jeffrey Patterson-Fortin, Troy E. Messick, Dan Feng, Niraj Shanbhag and Yingqun Wang, all from Penn.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to most recent data from the National Institutes of Health, received over $379 million in NIH research funds in the 2006 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s top ten “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.