PHILADELPHIA – Researchers at the University of Pennsylvania School of Medicine have engineered transplantable living nerve tissue that encourages and guides regeneration in an animal model. Results were published this month in Tissue Engineering.
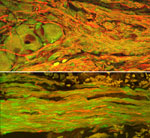 |
A surviving cluster of transplanted neurons at the graft extremity (top) with axons in the center (bottom). In both images, transplanted nerve cells are labeled green and axons are stained red. These axons are a mix of the transplanted axons and host axons, which intertwined as regeneration occurred directly across the transplanted tissue.
Click on thumbnail
to view full-size image |
About 300,000 Americans suffer peripheral nerve injuries every year, in many cases resulting in permanent loss of motor function, sensory function, or both. These injuries are a common consequence of trauma or surgery, but there are insufficient means for repair, according to neurosurgeons. In particular, surgeons need improved methods to coax nerve fibers known as axons to regrow across major nerve injuries to reconnect healthy targets, for instance muscle or skin.
“We have created a three-dimensional neural network, a living conduit in culture, which can be transplanted en masse to an injury site,” explains senior author Douglas H. Smith, MD, Professor, Department of Neurosurgery and Director of the Center for Brain Injury and Repair at Penn. Smith and colleagues have successfully grown, transplanted, and integrated axon bundles that act as ‘jumper cables’ to the host tissue in order to bridge a damaged section of nerve.
Previously, Smith and colleagues have “stretch-grown” axons by placing neurons from rat dorsal root ganglia (clusters of nerves just outside the spinal cord) on nutrient-filled plastic plates. Axons sprouted from the neurons on each plate and connected with neurons on the other plate. The plates were then slowly pulled apart over a series of days, aided by a precise computer-controlled motor system.
These nerves were elongated to over 1 cm over seven days, after which they were embedded in a protein matrix (with growth factors), rolled into a tube, and then implanted to bridge a section of nerve that was removed in a rat.
“That creates what we call a ‘nervous-tissue construct’,” says Smith. “We have designed a cylinder that looks similar to the longitudinal arrangement of the nerve axon bundles before it was damaged. The long bundles of axons span two populations of neurons, and these neurons can have axons growing in two directions - toward each other and into the host tissue at each side."
The constructs were transplanted to bridge an excised segment of the sciatic nerve in rats. Up to 16 weeks post-transplantation, the constructs still had their pre-transplant shape, with surviving transplanted neurons at the extremities of the constructs spanned by tracts of axons.
Remarkably, the host axons appeared to use the transplanted axons as a living scaffold to regenerate across the injury. The authors found host and graft axons intertwined throughout the transplant region, suggesting a new form of axon-mediated axonal regeneration. “Regenerating axons grew across the transplant bridge and became totally intertwined with the transplanted axons,” says Smith
Axons throughout the transplant region showed extensive myelination, the fatty layer surrounding axons. What’s more, graft neurons had extended axons beyond the margins of the transplanted region, penetrating deep into the host nerve. Remarkably, the constructs survived and integrated without the use of immunosuppressive drugs, challenging the conventional wisdom regarding immune tolerance in the peripheral nervous system.
The researchers suspect that the living nerve-tissue construct encourages the survival of the supporting cells left in the nerve sheath away from the injury site. These are cells that further guide regeneration and provide the overall structure of the nerve.
“This may be a new way to promote nerve regeneration where it may not have been possible before,” says co-first author D. Kacy Cullen, PhD, a post doctoral fellow in the Smith lab. “It’s a race against time - if nerve regeneration happens too slowly, as may be the case for major injuries, the support cells in the extremities can degenerate, blunting complete repair. Because our living axonal constructs actually grow into the host nerve sheath, they may ‘babysit’ these support cells to give the host more time to regenerate.”
The other co-first author is Jason Huang, MD, Assistant Professor of Neurosurgery at Rochester University, who participated in the study during his Neurosurgical residency at Penn.
This work was funded by the National Institutes of Neurological Disorders and Stroke and the Sharpe Trust.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to most recent data from the National Institutes of Health, received over $379 million in NIH research funds in the 2006 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s top ten “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.