PHILADELPHIA – They say you can put your foot in your mouth, and wear your heart on your sleeve. But your esophagus in your intestine? Researchers at the University of Pennsylvania School of Medicine have done just that – sort of.
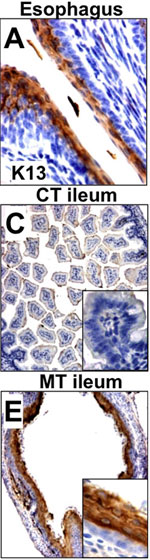 |
Cdx2-deficient intestine resembles the esophagus
Click on thumbnail
to view full-size image and description |
In a finding that helps resolve a long-standing question in developmental biology, Klaus H. Kaestner, PhD, Professor of Genetics, and colleagues report in the journal Developmental Cell this week about how the mammalian gut forms. Mice were genetically engineered to lack the protein Cdx2 in the cells that normally go on to form the stomach and intestine. The mutant animals – which invariably die either before or just after birth – have an esophagus where these missing organs should be.
The findings point to a potential genetic cause of a rare human congenital defect called colonic atresia, in which the colon is absent, giving pediatricians a candidate gene to study. Cdx2 is also implicated in a type of intestinal cancer.
“Our findings would suggest that either mutations in the Cdx2 gene itself or mutations in downstream targets controlled by Cdx2 could contribute to human colonic atresia,” says Kaestner.
Early in the development of mammal embryos, a structure called the primitive gut tube forms from a sheet of cells called the endoderm. Initially an unremarkable tube, this structure goes on to produce the esophagus, stomach, intestines, and colon, as well as associated organs such as the lungs, liver and pancreas. To develop these organs, the primitive gut tube needs to be organized, or patterned, along the long axis of the body. The question is, what signals help establish this length-wise pattern in the gut?
Several lines of evidence pointed to Cdx2, Kaestner says. First, the gene is expressed only in the intestine, with a sharp boundary between the stomach, which does not express Cdx2, and the duodenum of the intestine, which does.
In addition, Cdx2 expression is found in the esophagus of patients with intestinal metaplasia, a precancerous condition in which esophageal epithelial cells take on the characteristics of their intestinal counterparts. In fact, Kaestner and Debra Silberg, MD, formerly an Assistant Professor in Penn’s Division of Gastroenterology, had previously shown that forced expression of Cdx2 in the mouse stomach partially converts it to intestine, demonstrating that Cdx2 can cause intestinal metaplasia.
The present study asked a different question: What would happen if Cdx2 was absent during gut development? Other researchers had previously found that silencing Cdx2 in the entire mouse causes embryos to die early, precluding analysis of the gene’s role in gut formation. Kaestner and his team had to get creative to be able to answer these questions about early development.
Using conditional knockouts, the group effectively abolished Cdx2 expression in the embryonic gut with surgical precision and timing. The resulting animals, which Kaestner calls “designer mosaics,” express Cdx2 throughout the body until day 8.5 of embryogenesis, at which point the Cdx2 gene is silenced only in the developing gut; the rest of the body continues expressing the gene as before.
When the team studied the mosaic mice, Kaestner says, they found that “the intestine essentially forgets that it is supposed to be intestine and adopts a new cell fate. It actually looks like an esophagus.”
That, he explains, suggests that the default pattern of gut development is to form esophagus, and that Cdx2 acts to instruct the cells to instead become an intestine.
According to Kaestner, the findings were something of a surprise. Cdx2 is a transcription factor -- a protein that regulates the expression of other intestine-associated genes. It is one of three related proteins in the mice; the others are Cdx1 and Cdx4. Cdx1 is also expressed in the intestine, and Kaestner suspected the two proteins may serve redundant functions – that is, that Cdx1 would cover for the loss of Cdx2, and visa versa.
Instead, what his team discovered is that Cdx2 is the master regulator of intestinal development, serving to turn on Cdx1 as well as other genes, such as HNF1alpha and HNF4alpha. Each of these targets, like Cdx2, is also a transcription factor, with specific gene targets of their own. In other words, as with many other developmental programs, intestinal development occurs via the concerted action of a network of proteins that orchestrate transcription, with Cdx2 sitting right at the top.
“This finding helps us to understand how organ development in an embryo really occurs,” Kaestner says.
Kaestner’s team is now busy probing the function of Cdx2 at other developmental timepoints, as well as in colorectal cancer. Cdx2 may act to thwart carcinogenesis and they are using the mosaic mice to address that question.
The present study was funded by the National Institutes of Health.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to the National Institutes of Health, received over $366 million in NIH grants (excluding contracts) in the 2008 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s top ten “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.