PHILADELPHIA – Researchers at the University of Pennsylvania School of Medicine have found an association between the genetics of donor-recipient matches in kidney transplants and complications during the first week after transplantation. The team, led by Malek Kamoun MD, PhD, Professor of Pathology and Laboratory Medicine and Director of the Clinical Immunology and Histocompatibility Laboratory, and Harold Feldman MD, MSCE, Professor of Medicine and Epidemiology, has shown that small differences in the building blocks of cell-surface proteins used to match donors and recipients for deceased-donor kidney transplantation was associated with an increased risk for delayed allograft function, or DGF.
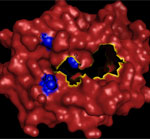 |
The three-dimensional structure mapping of the amino acid sequence in the antigen binding domain of the HLA-A molecule.
Click on thumbnail
to view full-size image |
The investigators published their findings this week online in the Proceedings of the National Academy of Sciences.
DGF is a common (30 to 50 percent incidence), but severe, malfunction of the transplant requiring dialysis in the first week after implantation, and has been associated with an increased risk of allograft rejection. In 2007, there were over 10,500 deceased-donor kidney transplants in America, according to the United Network for Organ Sharing.
“If we can validate the association we found in a larger dataset, then the genetic data can potentially be used to better match donors and recipients, thereby decreasing the level of immunosuppressants needed,” notes Kamoun. “The ultimate goal is to overcome the need for strong immunosuppressants. With immunosuppression comes an increased risk for infection and cancer, among other significant complications.”
The cause of DGF is poorly understood, with both non-immunological factors such as cause of death, donor age, recipient race, and immunological factors, playing a role. “But most studies haven’t clearly shown an association between the HLA-specific cell-surface proteins and DGF,” says Kamoun. “We have shown that amino acid variations in HLA-A proteins are associated with DGF.”
HLA cell-surface proteins allow the body to identify itself to avoid autoimmune reactions. Every person has a unique HLA signature. HLA proteins also identify foreign invaders in the body. “Their primary purpose is to distinguish, self versus non-self,” explains Kamoun. “That’s why we need to immunosuppress recipients following transplantation.”
The researchers looked at genetic variations in recipient and donor HLA proteins from blood samples (blood cells express HLA proteins) taken from 697 kidney transplant recipients and their deceased donors to obtain paired samples.
HLAs come in a variety of forms, differing by the sequence of their building blocks, or amino acids, from individual to individual. “Donor-recipient matching is based on genetic variations in HLA proteins,” says Kamoun. “By examining the HLA amino acid sequences of donor-recipient pairs, we asked: Are these genetic differences significantly associated with the recipient’s experience of DGF?”
Out of 66 amino-acid variations in HLA-A proteins, they found 15 were more strongly associated with DGF. They then focused on those, and taking other non-immunological factors into account, narrowed the important differences down to three amino acids that were highly associated with DGF. These are amino acids that play an important role in how pieces of foreign proteins are presented to immune cells. HLA proteins display these foreign molecules to specific recipient immune cells, called T lymphocytes.
Next steps for the project are to use a larger sample size from the UNOS database. Instead of looking at several hundred, they plan to look at thousands of donor-recipient pairs to validate their findings. “We also expect to see that these critical amino acids for DGF will vary with race,” predicts Kamoun. Future studies will also include the evaluation of other clinical outcomes such as acute rejection and graft loss.
John H. Holmes, Jane D. Kearns, Valerie Teal, Wei Peter Yang, Sylvia E. Rosas, Marshall M. Joffe, and Hongzhe Li, all of Penn, are co-authors. Ajay Israni, University of Minnesota, is also a co-author.
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to most recent data from the National Institutes of Health, received over $379 million in NIH research funds in the 2006 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s top ten “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.