PHILADELPHIA – Researchers at the University of Pennsylvania School of Medicine and colleagues in the United Kingdom have engineered T cells able to recognize HIV-1 strains that have evaded the immune system. The findings of the study, published online in the journal Nature Medicine, have important implications for developing new treatments for HIV, especially for patients with chronic infection who fail to respond to antiretroviral regimens.
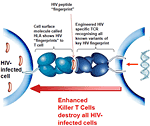 |
Killer T cells given a new version of the natural T cell receptor (TCR) are able to recognize all versions of a key HIV molecular fingerprint on the surface of infected cells and clear HIV infection in the laboratory cell cultures.
Credit: Adaptimmune Ltd, UK
Click on thumbnail
to view full-size image |
When viruses enter the body, they hijack the machinery of host cells to replicate and spread infection. When the body’s cells are infected with a virus they expose small parts of the virus on their surface, offering a molecular fingerprint called an epitope for killer T-cells from the immune system to see. This triggers an immune response, eliminating the virus and any cells involved in its production. However, HIV has the ability to mutate quickly, swiftly disguising its fingerprints to allow it to hide from killer T-cells.
Natural T cells recognize their targets through weak molecular interactions mediated by the T cell receptor. Through a clever molecular process, the investigators were able to isolate a group of T cell receptor encoding genes that bind to HIV-1 about 450-fold more strongly.
“Not only could T cells engineered to express the strongly binding T cell receptor see HIV strains that had escaped detection by natural T cells, but the engineered T cells responded in a much more vigorous fashion so that far fewer T cells were required to control infection,” says co-senior author James Riley, PhD, Research Associate Professor of Pathology and Laboratory Medicine at Penn.
 |
The Penn team, from left: Angel Varela-Rohena, PhD; James Riley, PhD; and Carl June, MD.
Credit: Coral Haas, University of Pennsylvania School of Medicine
Click on thumbnail
to view full-size image |
What’s more, adds first author Angel Varela-Rohena, PhD, who recently completed these studies as part of his PhD dissertation, “With the present availability of potent systems to replicate and deliver high-affinity HIV-1 specific T-cell receptors, billions of these anti-HIV-1 warriors can be generated in two weeks.”
“As soon as we saw over a decade ago how quickly the virus can evade the immune system we knew there would never be a conventional vaccine for HIV,” explains Professor Andy Sewell from Cardiff University, United Kingdom, co-senior author of the study. “In the face of our engineered assassin cells, the virus will either die or be forced to change its disguises again, weakening itself along the way. We’d prefer the first option but I suspect we’ll see the latter.”
“We hope to begin clinical trials using the engineered T cells in patients with advanced HIV infection next year, a group for whom many drug regimens have stopped working” says co-author Carl June, MD, Professor of Pathology and Laboratory Medicine and Director of Translational Research at the Abramson Family Cancer Research Institute at Penn. “If the therapy in that group proves successful, we will treat patients with early-stage, well-controlled HIV infection. The goal of these studies is to establish whether the engineered killer T cells are safe, and to identify a range of doses of the cells that can be safely administered.”
“We have managed to engineer a receptor that is able to detect HIV’s key fingerprints and is able to clear HIV infection in the laboratory,” says Bent Jakobsen, PhD, co-lead author and Chief Scientific Officer at Adaptimmune Ltd, the United Kingdom-based company which owns the rights to the technology. “If we can translate those results in the clinic, we could at last have a very powerful therapy on our hands.”
This study was funded in part by the National Institute of Allergy and Immune Diseases and Wellcome Trust, UK.
The Penn authors have no financial interest or other relationship with Adaptimmune LTD, apart from their scientific collaboration in developing the engineered killer T cell, conducting laboratory experiments and planning human clinical trials.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to most recent data from the National Institutes of Health, received over $379 million in NIH research funds in the 2006 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s top ten “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.