PHILADELPHIA – Researchers at the University of Pennsylvania School of Medicine have discovered that a protein called FOXA2 controls genes that maintain the proper level of bile in the liver. FOXA2 may become the focus for new therapies to treat diseases that involve the regulation of bile salts. The study was published online this week in Nature Medicine.
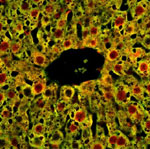 |
Liver cells from mice that do not express FOXA2.
Click on thumbnail
to view full-size image |
Bile, although made in the liver, is stored in the gall bladder and transported through ducts to the small intestine where it helps to digest fats from food. Bile salts, chemicals in bile that help digest fats and also keep cholesterol dissolved in the bile, are reabsorbed from the intestine and returned to the liver where they are broken up. The liver maintains a balance of bile salts by degrading old bile salts and synthesizing new ones. Problems arise when too many bile salts accumulate in the liver.
Diseases of bile regulation, such as primary sclerosing cholangitis (PSC), are characterized by problems with bile transport from the liver to the gut. The researchers found that in both children with biliary atresia and adults with PSC, syndromes of different etiologies, expression of FOXA2 in the liver is severely reduced. FOXA2 regulates expression of transporter proteins responsible for moving bile out of the liver, as well as several enzymes that function in bile acid detoxification. The study suggests that strategies to maintain FOXA2 expression might be a novel therapeutic goal.
In PSC, blockage of bile transport leads to inflammation of the bile duct and, over time, liver damage. The causes of PSC and related syndromes are not known, but may be autoimmune-related. PSC is associated with an increased risk of liver cirrhosis and liver cancer. Biliary atresia is a birth defect in which the bile ducts do not have normal openings, preventing bile from leaving the liver. The condition causes jaundice and cirrhosis of the liver.
Senior author Klaus Kaestner, PhD, Professor of Genetics, named the family of FOX genes and previous work in his lab showed that FOXA2 was important for glucose metabolism in the liver.
“Our interest in using genomics to study metabolic diseases led us to screen DNA from liver cells that expressed FOXA2 with an assay called ChIP on Chip,” explains first author Irina Bochkis, a doctoral student in Genomics and Computational Biology in the Kaestner lab, which resides in the Department of Genetics and the Institute for Diabetes, Obesity, and Metabolism at Penn.
ChIP-on-Chip assay stands for Chromatin ImmunoPrecipitation on a gene Chip.
First, in the two-step assay, chromatin (DNA and protein) was extracted from liver cells and an antibody specific for FOXA2 was used to recognize the protein and immunoprecipitate the FOXA2 protein-DNA complex. The antibody made a sandwich with FOXA2 in the middle and DNA on the sides. Then FOXA2 and its antibody were degraded, but the DNA that was bound to FOXA2 was put on a gene chip. The genes encoded by this DNA were then identified from this remaining DNA.
“We were surprised that a cluster of genes involved in lipid and steroid metabolism was identified by being bound to FOXA2,” says Bochkis.
This study also used mice that expressed no FOXA2 in their liver cells. Compared to normal littermates, the mutant mice accumulated bile salts and failed to detoxify them properly, which resulted in liver damage. In addition, liver biopsies from patients with PSC and biliary atresia, had no detectable levels of FOXA2. These findings suggest that low FOXA2 levels exacerbate liver injury.
Drug or DNA therapies that increase the expression of FOXA2 in liver cells may offer a new means of treating PSC and other similar syndromes. “In order to lay the groundwork for developing new treatments, we have to determine how FOXA2 itself is regulated,” notes Bochkis.
Co-authors are Nir Rubins, Peter White, and Emma Furth, of Penn and Joshua Friedman, of Children's Hospital of Philadelphia. The work was supported by the National Institutes of Health and a Penn Genomics Institute Graduate Fellowship.
###
PENN Medicine is a $3.5 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care. PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #4 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to most recent data from the National Institutes of Health, received over $379 million in NIH research funds in the 2006 fiscal year. Supporting 1,400 fulltime faculty and 700 students, the School of Medicine is recognized worldwide for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System includes three hospitals — its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation’s “Honor Roll” hospitals by U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center — a faculty practice plan; a primary-care provider network; two multispecialty satellite facilities; and home care and hospice.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.