| > |
Researchers at the University
of Pennsylvania School of Medicine have found an
absolute biochemical distinction between the sporadic and
hereditary variants of Lou Gehrig’s disease, or
amyothrophic lateral sclerosis (ALS), suggesting
that current approaches to drug discovery should be re-examined. |
| > |
By examining the various forms of ALS
in post-mortem tissue, the researchers found that TDP-43
was the disease protein in sporadic ALS cases, but not in
patients with SOD-1 mutations, all of whom have the familial
form of ALS. Patients with the SOD-1 mutation account for
about 1 percent of all ALS cases. |
| > |
This finding may partially account for
why therapeutic strategies, shown to be effective in SOD-1
mouse models, have generally not been effective in clinical
trials of sporadic ALS patients. |
| > |
The study was published this week in
the Annals of Neurology. |
(PHILADELPHIA) – Most research on Lou
Gehrig’s disease therapeutics has been based on the assumption that its two forms
(sporadic and hereditary) are similar in their underlying cause.
Now, researchers at the University of Pennsylvania
School of Medicine have found an absolute biochemical distinction between these two
disease variants, suggesting that current approaches to drug discovery
should be re-examined.
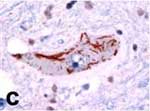 |
TDP-43 disease protein in amyotrophic
lateral sclerosis (ALS) brain tissue
Click on thumbnail
to view full-size image |
About 5 percent of all cases of Lou Gehrig’s disease, or
amyothrophic
lateral sclerosis (ALS), are passed from generation
to generation. The most common genetic variant in this familial
form is caused by a mutation in the SOD-1 gene. The researchers
looked at a large set of ALS patients, including hereditary cases,
both with and without the SOD-1 mutation.
The present study – published this week in the Annals
of Neurology – was conducted by Penn; a group led by Ian
Mackenzie from the University of British Columbia; the University
of Munich;
and others across the U.S. and Canada.
“Most ALS research has focused on how mutant SOD-1 proteins are toxic to nerve cells,” says senior author John
Trojanowski, MD, PhD, who directs the Penn Institute
on Aging. Last year, Penn
investigators led by co-author Virginia Lee,
PhD, who directs the
Penn Center for Neurodegenerative
Disease Research, identified
TDP-43 as the major disease protein in sporadic (non-hereditary)
forms of ALS, which are not those caused by SOD-1 gene mutations.
By examining the various forms of ALS in post-mortem tissue, the
researchers found that TDP-43 was the disease protein in sporadic
ALS cases, but not in patients with SOD-1 mutations, all of whom
have the familial form of ALS. Patients with the SOD-1 mutation
account for about 1 percent of all ALS cases.
“We argue that SOD-1 ALS does not equal sporadic ALS,” says
Trojanowski. “If you pursue drug discovery focusing on SOD-1-mediated pathways of brain and spinal cord degeneration you may benefit
SOD-1-bearing patients, but not the vast majority of ALS patients
who have the sporadic form of this disorder with TDP-43 pathologies underlying the disease.”
“Motor
neuron degeneration in TDP-43 cases may result from
a different mechanism than cases with SOD-1 mutations, so this
form of ALS may not be the familial counterpart of sporadic ALS,” surmises
Lee.
“This may also partially account for why therapeutic strategies,
shown to be effective in SOD-1 mouse models, have generally not
been effective in clinical trials of sporadic ALS patients,” explains
Trojanowski. “This also sounds a cautionary note in all other
diseases in which you have familial and sporadic versions of the
disease because it will prompt researchers to ask if mouse models
for drug discovery are based on the correct mutations or disease
protein.”
This research was funded by the Canadian
Institutes of Health Research, the National
Institute on Aging, the German
Federal Ministry of Education and Research, The
Wellcome Trust (United Kingdom) and the UK
Medical Research Council.
Co-authors in addition to Trojanowski, Lee, and Mackenzie are
Eileen H. Bigio, Paul G. Ince, Felix Geser, Manuela Neumann, Nigel
J. Cairns, Linda K. Kwong, Mark S. Forman, John Ravits, Heather
Stewart, Andrew Eisen, Leo McClusky, Hans A. Kretzschmar, Camelia
M. Monoranu, J. Robin Highley, Janine Kirby, Teepu Siddique, and
Pamela J. Shaw.
###
PENN Medicine is a $2.9 billion enterprise
dedicated to the related missions of medical education, biomedical
research, and high-quality patient care. PENN Medicine consists
of the University of Pennsylvania School of Medicine (founded in
1765 as the nation's first medical school) and the University of
Pennsylvania Health System.
Penn's School of Medicine is ranked #2 in the nation for receipt
of NIH research funds; and ranked #3 in the nation in U.S. News
& World Report's most recent ranking of top research-oriented
medical schools. Supporting 1,400 fulltime faculty and 700 students,
the School of Medicine is recognized worldwide for its superior
education and training of the next generation of physician-scientists
and leaders of academic medicine.
The University of Pennsylvania Health System includes three hospitals,
all of which have received numerous national patient-care honors [Hospital
of the University of Pennsylvania; Pennsylvania Hospital, the nation's
first hospital; and Penn Presbyterian Medical Center]; a faculty practice
plan; a primary-care provider network; two multispecialty satellite
facilities; and home care and hospice.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.