Electronic Nose Device Proves
Effective for Diagnosing Pneumonia and Sinusitis
Penn researchers show effectiveness
of device in analyzing gases exhaled from the nose to
determine presence of common bacterial infections
(Philadelphia, PA) – Researchers at the University
of Pennsylvania School of Medicine have recently
completed three studies – the most comprehensive
and largest to date – that demonstrate the effectiveness
of an electronic nose device for diagnosing common respiratory
infections, specifically pneumonia and sinusitis. Doctors
hope that the device – called the Cyranose 320,
or e-nose – will provide a faster, more cost-effective
and easier-to-use method for accurately diagnosing pneumonia
and, as a result, help reduce over-prescription of antibiotics.
Their initial findings will be presented at the combined
annual meetings of otorhinolaryngology (ear, nose and
throat) experts – the Triologic Society and the
American Broncho-Esophagological Association –
on April 30th, 2004, in Phoenix, Arizona.
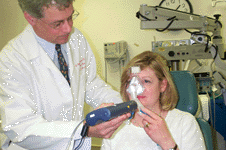 “Pneumonia
is a serious bacterial infection that can cause serious
injury or even death; indeed, it remains a leading cause
of death in intensive care units (ICUs),” said
lead author of the first study, C. William Hanson
III, MD, Professor of Anesthesia and board-certified
expert in critical care medicine (above, click on thumbnail
for more images). “Treating this illness is complicated
because there are many kinds of pneumonia, and it can
be commonly misdiagnosed in the ICU and confused with
other diseases which cannot be treated using antibiotics.
This is a leading cause of the overuse – through
over-prescription – of antibiotics for false cases
of pneumonia.”
“Pneumonia
is a serious bacterial infection that can cause serious
injury or even death; indeed, it remains a leading cause
of death in intensive care units (ICUs),” said
lead author of the first study, C. William Hanson
III, MD, Professor of Anesthesia and board-certified
expert in critical care medicine (above, click on thumbnail
for more images). “Treating this illness is complicated
because there are many kinds of pneumonia, and it can
be commonly misdiagnosed in the ICU and confused with
other diseases which cannot be treated using antibiotics.
This is a leading cause of the overuse – through
over-prescription – of antibiotics for false cases
of pneumonia.”
The first two studies looked at pneumonia cases among
patients who are on ventilators in the surgical intensive
care unit (SICU). Here, diagnosis is made difficult
by the patients’ limited ability to move, and
they are vulnerable to infections from other compounding
injuries. In the first study, researchers found that
the e-nose effectively diagnosed 92 percent of pneumonia
cases among 25 patients, as confirmed by computed tomography
(CT) scans of the lungs. It successfully distinguished
13 positive cases from 12 other patients who did not
have pneumonia. Similarly, in the second study, researchers
found the e-nose effective in providing accurate diagnoses
of pneumonia in 31 of 44 SICU patients (70 percent).
One quarter of ventilated SICU patients develop pneumonia
– a serious complication that can threaten the
patient’s life, requires immediate treatment with
antibiotics, and also increases their hospital stay
three-fold, with average additional hospital costs of
$11,000 per patient.
The third study looked at sinusitis, the most common
diagnosis from respiratory complaints by patients in
outpatient clinics. The e-nose was effective at diagnosing
82 percent of sinusitis cases among 22 patients, one
half infected and the other half not so.
All bacteria, as living organisms, produce unique arrays
or mixtures of exhaled gases. The e-nose works by comparing
“smellprints” from a patient’s breath
sample to standardized, or known, readings stored on
a computer chip. These “smellprints” are
created from both electro-chemical and mathematical
analysis of exhaled gases contained in a breath sample.
Upon analysis, identifiable patterns emerge, and a patient’s
“smellprint” can tell a physician whether
or not bacteria are present and, if so, what kind. This
can aid not just in the accuracy of diagnosis, but can
also help physicians select the most effective antibiotic
for treatment.
“The results confirm that exhaled breath can
be analyzed for pneumonia and sinusitis using a commercially
available e-nose device,” said lead investigator
for the sinusitis study and co-investigator for the
pneumonia studies, Erica Thaler, MD,
an Associate Professor of Otorhinolaryngology: Head
and Neck Surgery at Penn. “There is the potential
with this device to radically change and improve the
way we diagnose and treat both conditions – for
which there is no gold-standard test. And, given that
we can apply this sensory analysis to the detection
of pneumonia and sinusitis, then, hopefully, it can
be applied to common bacterial infections of the upper
respiratory tract.”
The e-nose is also being studied for its possible use
in diagnosing many other illnesses, including: lung
cancer, kidney disease and cirrhosis of the liver, otitis
media (middle ear infections) in children, or even detection
of chemicals and biological agents. Manufactured by
Smiths Detection of Pasadena, CA, the machines cost
approximately $8,000 USD, and still require approval
from the federal Food and Drug Administration before
they can be widely used. Breath samples are taken with
a hand-held sensor – about the size of child’s
video game player – connected to a standard oxygen
mask with cup, as the patient breathes normally. Readings
are displayed by connecting the device to a laptop computer.
"Flexibility and ease-of-use are the greatest advantages
of the e-nose," said lead researcher Neil Hockstein,
MD, a clinical instructor and Penn otorhinolaryngologist.
"They are miniaturized devices, provide quick results,
are relatively inexpensive, non-invasive, safe for patients
and they could be used in a doctor's office –
or, potentially, even at home."
For
a printer friendly version of this release, click
here.
# # #
Editor's note:
None of the three researchers – Drs. Hanson,
Thaler or Hockstein – have any financial interest
in Smiths Detection, or other electronic nose manufacturers.
Smiths Detection provided a small portion of study
funding for time provided by a research nurse. The studies
were primarily funded by the University of Pennsylvania
School of Medicine.
PENN Medicine is a $2.5 billion
enterprise dedicated to the related missions of medical
education, biomedical research, and high-quality patient
care. PENN Medicine consists of the University of Pennsylvania
School of Medicine (founded in 1765 as the nation's
first medical school) and the University of Pennsylvania
Health System (created in 1993 as the nation's first
integrated academic health system).
Penn's School of Medicine is ranked #2 in the nation
for receipt of NIH research funds; and ranked #4 in
the nation in U.S. News & World Report's most recent
ranking of top research-oriented medical schools. Supporting
1,400 fulltime faculty and 700 students, the School
of Medicine is recognized worldwide for its superior
education and training of the next generation of physician-scientists
and leaders of academic medicine.
Penn Health System consists of four hospitals (including
its flagship Hospital of the University of Pennsylvania,
consistently rated one of the nation's "Honor Roll"
hospitals by U.S. News & World Report), a faculty practice
plan, a primary-care provider network, three multispecialty
satellite facilities, and home health care and hospice.
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, excellence in patient care, and community service. The organization consists of the University of Pennsylvania Health System and Penn’s Raymond and Ruth Perelman School of Medicine, founded in 1765 as the nation’s first medical school.
The Perelman School of Medicine is consistently among the nation's top recipients of funding from the National Institutes of Health, with $550 million awarded in the 2022 fiscal year. Home to a proud history of “firsts” in medicine, Penn Medicine teams have pioneered discoveries and innovations that have shaped modern medicine, including recent breakthroughs such as CAR T cell therapy for cancer and the mRNA technology used in COVID-19 vaccines.
The University of Pennsylvania Health System’s patient care facilities stretch from the Susquehanna River in Pennsylvania to the New Jersey shore. These include the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, Chester County Hospital, Lancaster General Health, Penn Medicine Princeton Health, and Pennsylvania Hospital—the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Medicine at Home, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is an $11.1 billion enterprise powered by more than 49,000 talented faculty and staff.