Developing new cancer treatments requires years of research and thousands of hours of dedicated effort. But many new therapies still never meet the FDA's strict safety and efficacy standards for approval. Penn Medicine has a proven track record of cancer research and discovery, with 21 FDA approvals for drugs and techniques to treat cancer since 2017. Eight of these therapies were the first treatment options available for patients with a specific disease.
We are proud to say that these discoveries made at Penn Medicine have translated into cancer care now available to patients nationwide — and across the globe.
Read the full list of Penn Medicine's FDA approved cancer therapies
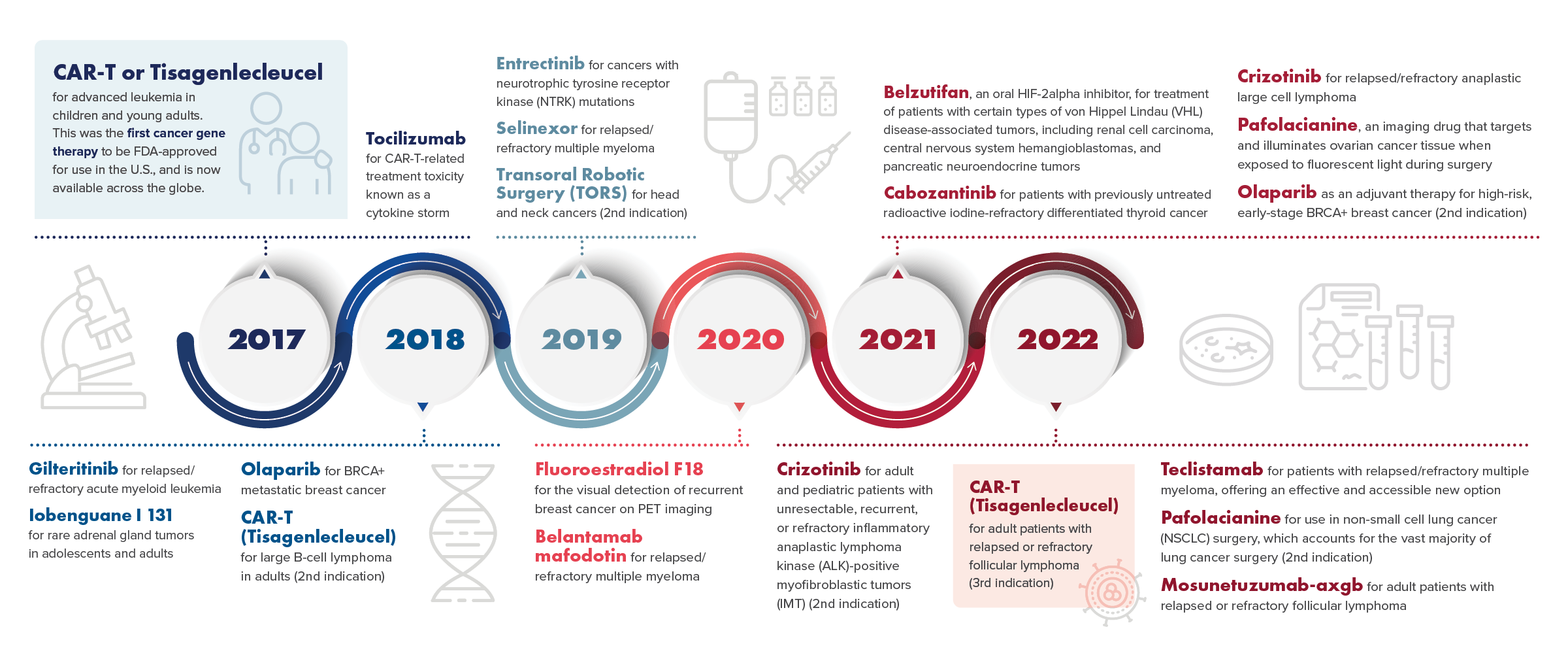
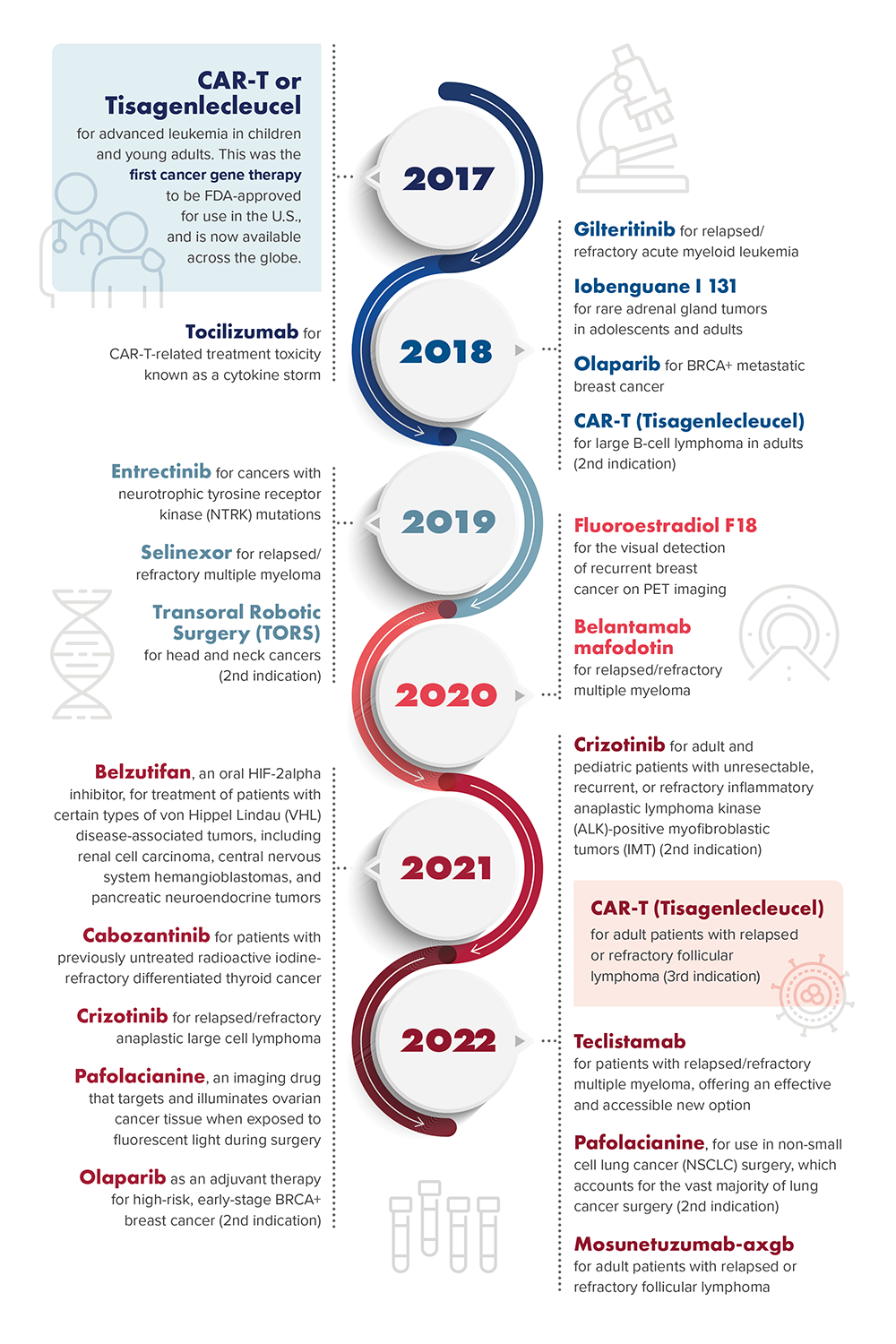
Penn Medicine's Recent FDA-Approved Cancer Treatments and Therapies
-
Tisagenlecleucel (Kymriah) is the first personalized gene therapy in the United States for:
- Children and adults with relapsed/refractory B-cell acute lymphoblastic leukemia (2017.)
- Adults with relapsed and refractory aggressive B-cell lymphoma (2018.)
- Adults with relapsed/refractory follicular lymphoma (2022.)
- Tocilizumab (Actemra) treats side effects from cancer immunotherapy (2017.)
- Olapirib (Lynparza) targets BRCA-mutated refractory ovarian cancer and metastatic breast cancer (2018) and early-stage breast cancer with BRCA1/2 mutations (2022.)
- Iobenguane I 131 (Azedra) is the first and only treatment option for adults and children with advanced and inoperable pheochromocytomas and paragangliomas (2018.)
- Gilteritinib (Xospata) is the first drug of its kind for relapsed or refractory FLT3-mutated acute myeloid leukemia (2018.)
- Transoral Robotic Surgery (TORS) was approved as a technology for operating on head and neck cancers (2019.)
- Selinexor (Xpovio) is the first treatment of its kind for relapsed/refractory multiple myeloma (2019.)
- Entrectinib (Rozlytrek) targets NTRK-mutated cancers in children and adults and ROS1-mutated non-small-cell lung cancers in adults (2020.)
- Fluoroestradiol F 18 (Cerianna) enables visual detection of recurrent breast cancer by PET scan (2020.)
- Belantamab mafodotin-blmf (Blenrep) is the first antibody – drug conjugate that selectively targets and kills myeloma cells to treat relapsed/refractory multiple myeloma (2020.)
- Crizotinib (Xalkori) treats relapsed/refractory ALK-mutated systemic anaplastic large-cell lymphoma as well as inflammatory ALK-mutated myofibroblastic tumors in children and young adults (2021-22.)
- Belzutifan (Welirig) is the first therapy of its kind for treating von Hippel – Lindau disease-associated tumors, such as renal cell carcinoma, central nervous system hemangioblastomas and pancreatic neuroendocrine tumors (2021.)
- Cabozantinib (Cabometyx) treats refractory differentiated thyroid cancer in children and adults (2021.)
- Pafolacianine (Cytalux) is the first FDA-approved substance to illuminate ovarian cancer (2021) and lung cancer (2022) lesions during surgery.
- Teclistamab-cqvy (Tecvayli) is the first bispecific T cell engager antibody for the treatment of patients with relapsed or refractory multiple myeloma (2022.)
- Mosunetuzumab-axgb (Lunsumio) is a bispecific CD20-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma after two or more lines of systemic therapy (2022.)